On a sweltering summer afternoon, few things bring as much instant delight as a scoop of perfectly chilled ice cream. Yet, this fleeting joy is often cut short by a familiar, dripping disappointment. The phenomenon of ice cream melting, a source of minor culinary tragedy for centuries, is not merely a matter of temperature but a fascinating dance of physics and chemistry centered on a principle known as freezing point depression. This scientific concept transforms a simple mixture of cream, sugar, and flavorings into a complex, structured delight that is inherently unstable against the warmth of a summer day.
The journey of ice cream begins not in the freezer but in the formulation of its base. At its core, ice cream is an intricate colloid, a mixture where one substance of microscopically dispersed particles is suspended throughout another. It is simultaneously a foam (with air bubbles whipped in), an emulsion (with tiny fat globules dispersed in water), and a solution (with sugar and salts dissolved in water). This complex structure is what gives ice cream its beloved texture, but it is also the very reason for its thermodynamic vulnerability. The key player in its instability is the dissolved sugar, which performs a crucial function during freezing but becomes an agent of its demise upon warming.
The magic and the misery both start with the freezing point. Pure water freezes at 0 degrees Celsius (32 degrees Fahrenheit). However, when you dissolve a solute like sugar (sucrose) in water, the freezing point of the resulting solution drops. This is freezing point depression, a colligative property, meaning it depends on the number of dissolved particles in a solvent, not their identity. In an ice cream mix, the high concentration of sugar molecules, along with dissolved salts from the milk solids, interferes with the water molecules' ability to form the rigid crystalline structure of ice. The water requires a colder environment to finally solidify. This is why your home freezer, typically set around -18°C (0°F), is cold enough to freeze the water in the mix into a vast number of tiny ice crystals, creating a semi-solid foam rather than a solid block.
This depression is a double-edged sword. On one hand, it is essential for creating a scoopable, soft texture. If the freezing point weren't depressed, the mixture would freeze solid into an impenetrable block of ice, much like a ice pop without a stick, making it impossible to scoop and unpleasant to eat. The sugar ensures that even at the low temperature of the freezer, not all the water is frozen; a significant portion remains as a thick, syrupy liquid surrounding the microscopic ice crystals and air bubbles. This unfrozen phase, rich in sugar, is what provides the smooth, creamy mouthfeel we cherish. It acts as a lubricant, allowing the ice crystals to slide past one another instead of locking together.
However, this very same unfrozen phase is the Achilles' heel of ice cream. When you remove the carton from the freezer, it begins to absorb heat energy from its surroundings. This energy does not immediately raise the temperature of the ice cream; instead, it goes into melting the network of tiny ice crystals. The process of melting requires energy, known as the latent heat of fusion, which buffers the temperature rise momentarily. But as melting proceeds, the concentration of sugar in the remaining liquid phase changes dynamically, further altering the local freezing point in a cascading effect.
As the most exposed parts of the scoop warm up, the ice crystals melt, releasing liquid water. This water dilutes the surrounding sugar syrup, which in turn slightly raises its freezing point. However, the ambient temperature is still far above this new, slightly elevated freezing point, so melting accelerates. The structure begins to collapse. The air bubbles, once stabilized by a matrix of partially frozen cream and fat, coalesce and escape. The fat globules, which were also helping to stabilize the entire structure, start to clump together and separate in a process known as buttering. The once smooth emulsion breaks, and the mixture transitions from a solid foam to a viscous, watery liquid—the sad puddle at the bottom of a cone.
The rate of this meltdown is not uniform and is influenced by the ice cream's specific composition. A higher sugar content will depress the freezing point further, meaning the product might actually melt slightly faster because a greater proportion of its water is unfrozen even at very low temperatures, making the structure less stable from the outset. The type of sugar matters too. Sucrose is common, but many commercial producers use corn syrup or other sugars. Corn syrup contains long-chain glucose polymers that can interfere more effectively with ice crystal formation and recrystallization, potentially offering a slightly slower melt by providing more structural viscosity in the unfrozen phase, but it can also impact the sweetness and mouthfeel.
Furthermore, modern stabilizers and emulsifiers like guar gum, carrageenan, and lecithin are added to combat this very issue. They work by binding water, preventing large ice crystals from forming (which makes ice cream grainy), and helping to maintain the air bubble structure for longer. They effectively increase the viscosity of the unfrozen phase, making it thicker and more resistant to flow. This is why some premium or heavily stabilized ice creams seem to melt more slowly and maintain their shape for a longer period before ultimately succumbing to the inevitable collapse. They don't prevent melting; they simply slow the structural failure, creating a thicker, more cohesive melt rather than a thin, milky soup.
The environmental conditions play an equally brutal role. Ambient temperature is the obvious factor, but humidity is a stealthy accomplice. On a humid day, the air is saturated with water vapor, which reduces the rate of evaporation. As ice cream melts, a slight cooling effect occurs from evaporation. On a dry day, this evaporation happens more readily, providing a small cooling effect that can slightly slow the melting process. In high humidity, this evaporative cooling is minimized, allowing the ice cream to warm and melt unimpeded. The material of the container also matters; a warm ceramic bowl will transfer heat into the dessert far more quickly than a thermally insulating waffle cone.
For the consumer, the race against time is a familiar one. The strategies are intuitive: eat quickly, seek shade, or, for the truly dedicated, pre-chill the bowl. For the food scientist, the quest is eternal—to formulate the perfect balance of ingredients that delivers sublime flavor and texture while offering just a few more minutes of grace under the sun. It is a battle against fundamental thermodynamics, a fight to temporarily suspend the inevitable transition from order to disorder.
So, the next time you find yourself frantically licking a rivulet of sweet cream running down your hand, remember you are witnessing a complex physical chemistry experiment in real-time. That sticky sweetness is the direct result of freezing point depression, the very same principle that gave you a soft, scoopable treat now betraying you under the sun's relentless heat. It is, indeed, a sweet烦恼—a delightful烦恼, but a烦恼 nonetheless, reminding us that even the simplest pleasures are governed by the profound and inescapable laws of nature.
In the quiet corners of libraries, the bustling floors of tech startups, and the focused atmosphere of university common rooms, a small, colorful object has become an unexpected symbol of cognitive harmony. The Rubik's Cube, a puzzle invented in 1974 by Hungarian sculptor and professor of architecture Ernő Rubik, has long transcended its status as a mere toy. It has evolved into a profound tool for understanding the intricate dance between spatial reasoning, pattern recognition, and the synchronized thinking required to conquer a complex, three-dimensional challenge.
On a sweltering summer afternoon, few things bring as much instant delight as a scoop of perfectly chilled ice cream. Yet, this fleeting joy is often cut short by a familiar, dripping disappointment. The phenomenon of ice cream melting, a source of minor culinary tragedy for centuries, is not merely a matter of temperature but a fascinating dance of physics and chemistry centered on a principle known as freezing point depression. This scientific concept transforms a simple mixture of cream, sugar, and flavorings into a complex, structured delight that is inherently unstable against the warmth of a summer day.
It was a classic scene in countless films and novels: two characters find themselves in a precarious situation, perhaps hanging from a crumbling ledge or fleeing from some imminent danger. Their hearts pound, their palms sweat, and in the heightened state of shared terror, a powerful, often unexpected, romantic connection sparks between them. For decades, this was dismissed as a convenient plot device, a fanciful notion with little basis in reality. However, the intriguing psychological phenomenon now known as the "Suspension Bridge Effect" suggests there might be a profound scientific truth buried within this cinematic trope, revealing a fascinating and complex link between our physiological arousal and the experience of romantic attraction.
In an age where smartphones have become ubiquitous extensions of our daily lives, their capabilities extend far beyond communication and entertainment. One of the most overlooked yet potentially life-saving features lies in the humble LED flash—a component typically associated with photography that has quietly evolved into an emergency signaling tool. The concept of using light as a distress signal is ancient, but its integration into modern handheld devices represents a fascinating convergence of tradition and technology.
Beneath the ocean's shimmering surface lies one of Earth's most magnificent and paradoxical creations—coral reefs. These vibrant underwater metropolises, often dubbed the rainforests of the sea, are not merely collections of colorful organisms but are, in fact, monumental feats of natural architecture. At the heart of their existence is a silent, relentless, and ancient promise: the perpetual accumulation of calcium carbonate. This process, both delicate and mighty, constructs the very foundations upon which entire ecosystems thrive, supporting an astonishing quarter of all marine life. The story of coral growth is a narrative written in stone, a testament to persistence that spans millennia, yet it is a narrative now under threat, making its understanding more critical than ever.
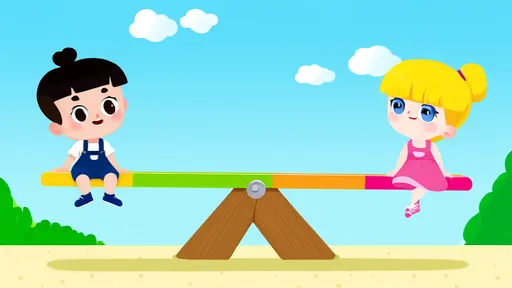
In the heart of every playground, there exists a simple yet profound teacher of physics—the seesaw. This timeless apparatus, often taken for granted as mere child’s play, is a living demonstration of one of the most fundamental principles of mechanics: the lever. The joy of soaring into the air, suspended by the weight of a friend, is not just a moment of laughter but an experiential lesson in balance, force, and equilibrium.
In the world of philately, few elements are as functionally elegant and historically significant as the humble perforation. These tiny rows of holes, often overlooked by the casual observer, represent a revolutionary advancement in postal technology and a fascinating intersection of design, engineering, and commerce. The story of the postage stamp perforation is not merely a technical footnote; it is a narrative of how a simple innovation solved a practical problem and, in doing so, added a new layer of complexity and beauty to stamp collecting.
In the heart of a sweltering studio, where temperatures soar and the air shimmers with heat, an ancient dance unfolds between artisan and element. This is the world of glassblowing, a craft where fire and breath collaborate to shape molten silica into objects of breathtaking beauty and fragility. The process, often described as a form of alchemy, is more than mere technique; it is a profound metaphor for human connection, a narrative of love molded under intense pressure and radiant heat. The title Glassblowing: A Vessel of Love Forged in High Temperatures captures this essence perfectly, framing the art not just as a physical transformation but as an emotional and symbolic journey.
The celestial dome has long served as humanity's window to the cosmos, a architectural marvel that bridges terrestrial existence with celestial wonder. Modern planetariums have evolved from simple observation points to sophisticated theaters of astronomical education, where mathematical modeling transforms abstract cosmic principles into tangible visual experiences. The intricate dance of projecting accurate night skies onto domed surfaces represents one of the most fascinating applications of mathematical precision in public education, merging artistic vision with scientific rigor in ways that continue to captivate audiences worldwide.
In the dimly lit theater, a lone artist stands before a glowing light table, hands hovering over a thin layer of fine sand. The audience holds its breath as fingers begin to dance—sweeping, flicking, tracing—transforming inert grains into living stories. This is sand animation: an ephemeral art form where narratives bloom and vanish in the same breath, where permanence surrenders to poetry.
In the quiet hum of modern kitchens, a revolution has been simmering—one powered not by flames but by microwaves. The concept of microwave cooking often conjures images of hastily reheated leftovers or soggy frozen meals, yet beneath this misunderstood reputation lies a sophisticated science of molecular vibration heating that is reshaping how we approach gourmet dining for two. This isn’t about convenience alone; it’s about unlocking flavors, textures, and culinary creativity through the precise manipulation of energy.
In the heart of the city stands an ancient clock tower, its face weathered by centuries yet still keeping impeccable time. The mechanism within is a masterpiece of engineering, a symphony of interlocking gears that dance in perfect harmony. For generations, this tower has not only measured the hours but also symbolized the relentless pursuit of precision in a world governed by chaos.
In the quiet corners of autumn, when the world sheds its summer skin, a delicate artistry emerges from the fallen leaves. The concept of Leaf Collage: A Natural Love Letter from Plant Veins captures more than just an aesthetic pursuit—it is a meditation on the ephemeral beauty of nature and the stories etched into every fragile surface. Each leaf, with its intricate network of veins, carries the whispers of seasons passed, the resilience of growth, and the gentle surrender to decay. This practice transforms what many consider debris into a canvas, where the language of nature is spelled out in hues of amber, crimson, and gold.
In the quiet space between two bodies moving as one, there is a conversation that transcends words. Partner yoga, an ancient practice reimagined for modern connection, is far more than a series of synchronized stretches. It is a dynamic laboratory of human interaction, a living equation where the variables of weight, balance, and momentum are solved not with numbers, but with trust. The theme of Partner Yoga: The Trust Equation of Body Mechanics delves into this intricate dance, exploring how the principles of physics and the nuances of psychology intertwine to create a practice that builds strength, flexibility, and profound relational bonds.
In the quiet predawn hours, when the world still slumbers beneath a blanket of stars, navigators of old would consult their most trusted companion: the magnetic compass. Its needle, dancing with an almost sentient grace, would eventually settle, pointing unerringly toward the magnetic north. For centuries, this simple yet profound instrument has guided explorers across trackless oceans, through dense, uncharted forests, and over vast, featureless deserts. It is a symbol of human ingenuity, a tool that harnesses the very heartbeat of the planet to show us the way. The relationship between the traveler and the compass is one of pure faith—a belief that the needle's whisper is truth.